Steel is a combination of iron and carbon. In its softened state, the base is a matrix composed of simple iron molecules (ferrite), in which are suspended molecules of iron carbide (cementite). When steel is heated to prescribed temperatures, then cooled at a specific rate, it undergoes physical internal changes which manifest themselves in the form of various micro- structures such as pearlite, bainite and martensite. These micro-structures (and others) provide a wide range of mechanical properties, making steel and supremely versatile metal. Alloying elements are added to effect changes in the properties of steel. The basis of the article is to cover some of the different alloying elements added to the basic system of iron and carbon, and what they do to change the properties or effectiveness of steel.
Carbon.
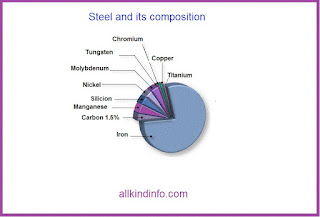
The presence of carbon in iron is necessary to make steel. Carbon is essential to the formation of cementite (as well as other carbides), and to the formation of pearlite, spheroidite,bainite and iron-carbon martensite, withe martensite being the hardest of the micro-structures, and the the structure sought after by knifemakers. The hardness of steel (or more accurately, the harden ability) is increased by the addition of more carbon, up to about 0.65 percent. Wear resistance can be increased in amounts up to about 1.5 percent. Beyond this amount , increases of carbon reduce toughness and increase brittleness. The steel of interest to knife makers generally contain between 0.5 and 1.5 percent carbon. They are described as follows.
Low Carbon Steel : Under 0.4 percent
Medium Carbon Steel : 0.4 To 0.6 percent
High Carbon Steel : 0.7 to 1.5 percent
Carbon is the single most important alloying element in the steel.
Manganese
Manganese slightly increase the strength of ferrite, and also increase the hardness penetration of steel in the quench by decreasing the critical quenching speed.This also makes the steel more stable in the quench. Steel with manganese can be quenched in oil rather than water, and therefore are less susceptible to cracking because of a reduction in the shock of quenching. Manganese is present in most commercially made steels.
Chromium
As with manganese, chromium has a tendency to increase hardness penetration. This element has many interesting effects on steel. When 5 percent chromium or more is used in conjunction with manganese, the critical quenching speed is reduced to the point that the steel becomes air hardening. Chromium can also increase the toughness of steel, as well as the wear resistance. Probably one of the most well know effects of chromium on steel is the tendency to resist stainless steels. A more accurate term would be stain resistant. Stainless tool steels will in fact darken and rust, just not as readily as the non-stainless varieties. Steels with chromium also have higher critical temperatures in heat treatment.
Silicon
Silicon is used as a deoxidizer in the manufacture of steel. It slightly increases the strength of ferrite, and when used in conjunction with other alloys can help increase the toughness and hardness penetration of steel.
Nickel
Nickel increase the strength of ferrite, therefore increasing the strength of the steel. It is used in low alloy steels to increase toughness and hardenability. Nickel also tends to help reduce distortion and cracking during the quenching phase of heat treatment.
Molybdenum
It increase the hardness penetration of the steel, slows the critical quenching speed and increase high temperature tensile strength.
Vanadium
Vanadium helps control gain growth during heat treatment. By inhibiting grain growth it helps increase the toughness and strength of steel.
Tungsten
Used in small amount, tungsten combines with the free carbides in steel during heat treatment, to produce high wear resistance with little or no loss of toughness.High amounts combined with chromium gives steel a property known as red hardness. An example of this would be tools designed to cut hard materials at high speeds, where the friction between the tool and the material would generate high temperature.
Copper
The addition of copper in amounts of 0.2 to 0.5 percent primarily improves steels resistance to atmospheric corrosion. It should be noted that with respect to knife steels, copper has a detrimental effect to surface quality and to hot-working behavior due to migration into the grain boundaries of the steel.
Niobium
In low carbon alloy steels niobium lowers the transition temperature and aids in a fine grain structure. Niobium retards tempering and can decrease the harden ability of steel because it forms very stable carbides. This can mean a reduction in the amount of carbon dissolved into the austenite during heat treatment.
Titanium
This element when used in conjunction with Boron, increases the effectiveness of the Boron in the harden ability of steel.
Silicon
Silicon is used as a deoxidizer in the manufacture of steel. It slightly increases the strength of ferrite, and when used in conjunction with other alloys can help increase the toughness and hardness penetration of steel.
Nickel
Nickel increase the strength of ferrite, therefore increasing the strength of the steel. It is used in low alloy steels to increase toughness and hardenability. Nickel also tends to help reduce distortion and cracking during the quenching phase of heat treatment.
Molybdenum
It increase the hardness penetration of the steel, slows the critical quenching speed and increase high temperature tensile strength.
Vanadium
Vanadium helps control gain growth during heat treatment. By inhibiting grain growth it helps increase the toughness and strength of steel.
Tungsten
Used in small amount, tungsten combines with the free carbides in steel during heat treatment, to produce high wear resistance with little or no loss of toughness.High amounts combined with chromium gives steel a property known as red hardness. An example of this would be tools designed to cut hard materials at high speeds, where the friction between the tool and the material would generate high temperature.
Copper
The addition of copper in amounts of 0.2 to 0.5 percent primarily improves steels resistance to atmospheric corrosion. It should be noted that with respect to knife steels, copper has a detrimental effect to surface quality and to hot-working behavior due to migration into the grain boundaries of the steel.
Niobium
In low carbon alloy steels niobium lowers the transition temperature and aids in a fine grain structure. Niobium retards tempering and can decrease the harden ability of steel because it forms very stable carbides. This can mean a reduction in the amount of carbon dissolved into the austenite during heat treatment.
Titanium
This element when used in conjunction with Boron, increases the effectiveness of the Boron in the harden ability of steel.

Post a Comment