Cast Iron
Pig iron alongside scrap iron, coke and limestone is liquefied in a vertical furnace called cupola. Here likewise the carbon and different impurities introduce oxidize in nearness of a little measure of air, to shape slag. The liquid iron got from dome heater can be thrown into forms. It is, thus, known as cast iron. It comprises of 93-94% Ferrous, 2-4% carbon and a small amount of S, P, Si and Mn polluting influences. It is hard and brittle. It is cast into covers of main holes, channel pipes, casings of machines etc.
Wrought Iron
The most purist form of iron is called wrought iron, which contains up to 99 % of iron and small amount of carbon with impurities such as sulfur , manganese, silicon and phosphorus.wrought iron is malleable, tough, gray and can be welded. It is mold able and has a sinewy structure because of the nearness of thin movies of slag between layers of pure iron. The nearness of slag makes it greatly extreme and safe towards rusting
Manufacturing Process
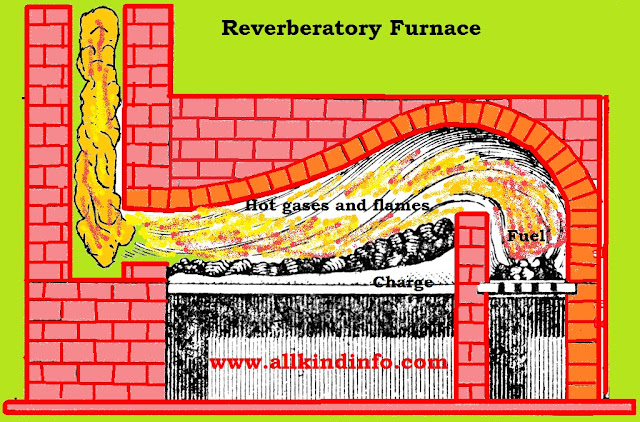
It is acquired from cast iron by evacuating the significant partition of its impurities influences by the surely understood puddling process.The cast iron alongside some scrap iron is warmed on the hearth of a reverberatory furnace lined with hematite. The hot gasses and flames reflected from the top of the heater falls upon the charge set on the hearth. The cast iron softens down and the liquid mass is blended or puddled at intervals by method for a long pole presented through a channel in the wall of the furnace. The haematite supplies the oxygen required to oxidize the carbon, sulfur, silicon, manganese and phosphorus show in the cast iron.
Chemical Reaction
Carbon and sulfur are oxidized to carbon dioxide and sulfur dioxide, individually. Silicon and manganese are oxidized to silica and manganese oxide, which join to frame manga-nous silicate.
3Si + 2Fe203 —* 3Si02 + 4Fe 3Mn + Fe203 —» 3MnO + 2Fe MnO + Si02 —» MnSi03
Slag Formation
As the contamination are dispensed with, the liquefying purpose of the metal ascents and iron gets to be pasty. The pasty mass is blended which frames "balls" or "blooms" which are elastic in composition because of a lot of slag. The balls are taken out from the heater and the slag is crushed out by crushing or hammering. At last, iron is moved into sheets or manufactured into bars.
Chemical Reaction
Phosphorus is oxidized to phosphorus pentoxide which forms ferric phosphate slag with haematite.
P205 + Fe203 —* 2FeP04

Post a Comment